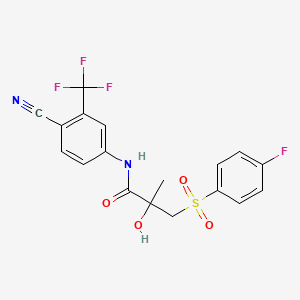
D0477 | bicalutamide
L
L02BB03 Bicalutamide
[L02BB] Anti-androgens
[L02B] HORMONE ANTAGONISTS AND RELATED AGENTS
[L02] ENDOCRINE THERAPY
[L] Antineoplastic and immunomodulating agents
L02AE51 Leuprorelin and bicalutamide
[L02AE] Gonadotropin releasing hormone analogues
[L02A] HORMONES AND RELATED AGENTS
[L02] ENDOCRINE THERAPY
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | decrease | 53 | |||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 265 companies from 18 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H315 (93.21%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (93.21%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (92.83%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (+-)-4'-Cyano-alpha,alpha,alpha-trifluoro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide | (2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide | 357B065 |
| 4'-cyano-3-[(4- fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide | 4'-cyano-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide | 90357-06-5 |
| A803039 | AB0008136 | AB00639963-06 |
| AB00639963-08 | AB00639963-09 | AB00639963_10 |
| AC-4232 | ACT06291 | AK544272 |
| AKOS015895073 | AOB5596 | API0001710 |
| AT-9158 | B3206 | BCP02110 |
| BCP9000408 | BCPP000337 | BDBM18525 |
| BRD-A29485665-001-03-7 | BRN 5364666 | Bicalutamide (CDX) |
| Bicalutamide (CDX), >=98% (HPLC), powder | Bicalutamide (Casodex) | Bicalutamide (JAN/USP/INN) |
| Bicalutamide - Casodex | Bicalutamide [USAN:INN:BAN] | Bicalutamide [USAN:USP:INN:BAN] |
| Bicalutamide for system suitability, European Pharmacopoeia (EP) Reference Standard | Bicalutamide(Casodex) | Bicalutamide, 97% |
| Bicalutamide, British Pharmacopoeia (BP) Reference Standard | Bicalutamide, European Pharmacopoeia (EP) Reference Standard | Bicalutamide, Pharmaceutical Secondary Standard |
| Bicalutamide, United States Pharmacopeia (USP) Reference Standard | Bicalutamine | C08160 |
| C18H14F4N2O4S | CB0180 | CCG-100951 |
| CCG-220876 | CCG-222330 | CCRIS 8728 |
| CHEBI:144093 | CHEBI:91617 | CHEMBL409 |
| CPD000466329 | CS-1296 | Calutide |
| Casodex | Casodex (TN) | Certified Reference Material |
| Cosudex | D00961 | DB-041165 |
| DB01128 | DTXSID2022678 | EX-A962 |
| FT-0080576 | FT-0618286 | GTPL2863 |
| HMS2051B13 | HMS2089N12 | HMS2232H03 |
| HMS3263M13 | HMS3372K05 | HMS3393B13 |
| HMS3654K18 | HMS3714P13 | HSDB 7655 |
| HY-14249 | ICI 176,334 | ICI 176334 |
| ICI-176334 | ICI176,334-1 | J10442 |
| KS-00000XEI | KS-1161 | Kalumid |
| LKJPYSCBVHEWIU-UHFFFAOYSA-N | LP01026 | LS-119125 |
| MFCD00869971 | MLS000759437 | MLS001424047 |
| N-(4-cyano-3-(trifluoromethyl)phenyl) | N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methylpropanamide | N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide |
| N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropionamide | N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide | N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-methyl-2-oxidanyl-propanamide |
| N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorobenzene)sulfonyl]-2-hydroxy-2-methylpropanamide | N-[4-cyano-3-trifluoromethyl-phenyl]-3-[4-fluorophenyl-sulfonyl]-2-hydroxy-2-methyl-propionamide | NC00201 |
| NCGC00167977-01 | NCGC00167977-02 | NCGC00167977-03 |
| NCGC00167977-09 | NCGC00261711-01 | NSC-722665 |
| NSC-759816 | NSC722665 | NSC759816 |
| PRO113 | Pharmakon1600-01504827 | Propanamide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-, (+-)- |
| Propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl- | PubChem18237 | Q1988832 |
| Raffolutil | SAM001246612 | SB17301 |
| SC-17332 | SCHEMBL3611 | SMR000466329 |
| SR-01000759410 | SR-01000759410-4 | SR-01000759410-5 |
| SW197581-4 | Tox21_501026 | bicalutamide |
| propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-,(+-); | s1190 |