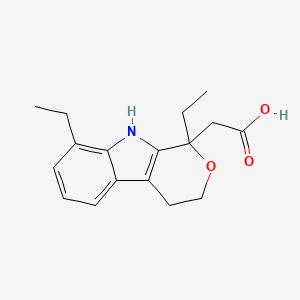
D0051 | Etodolac
M
M01AB08 Etodolac
[M01AB] Acetic acid derivatives and related substances
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | isolated mitochondria | increase | 56 | |||||
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 48 companies from 6 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H319 (89.58%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P280, P301+P310, P305+P351+P338, P321, P330, P337+P313, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (+-)-1,8-Diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic acid | (1,8-Diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indol-1-yl)-acetic acid | (1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic acid |
| (RS)-2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetic acid | 1,3,4,9-Tetrahydro-1,8-diethylpyrano(3,4-b)indole-1-acetic acid | 1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid |
| 1,8-Diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic acid | 1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid | 1,8-Diethyl-1,3,4,9-tetrahydropyranol[3,4-b]indole-1-acetic acid |
| 1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indole-1-acetic acid | 1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-ylacetic acid | 2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic acid |
| 2-(1,8-diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetic acid | 2-(1,8-diethyl-4,9-dihydro-3H-pyrano[3,4-b]indol-1-yl)acetic acid. | 2-{1,8-diethyl-1H,3H,4H,9H-pyrano[3,4-b]indol-1-yl}acetic acid |
| 340E254 | 41340-25-4 | AB00052194 |
| AB00052194_17 | AB0012214 | AC-4231 |
| AK550845 | AKOS015838610 | ANW-42024 |
| AY 24,236 | AY 24236 | AY 24236;AY-24,236 |
| AY-24,236 | AY-24236 | AY-24236 |
| AY24,236 | AY24236 | BCP28411 |
| BDBM50016799 | BPBio1_000333 | BRD-A74667430-001-05-3 |
| BRD-A74667430-001-15-2 | BSPBio_000301 | BSPBio_003138 |
| C06991 | CAS-41340-25-4 | CCG-39005 |
| CCRIS 3923 | CE0096 | CHEBI:4909 |
| CHEMBL622 | CPD000058443 | CS-0832 |
| CTK4I4719 | D00315 | DB00749 |
| DSSTox_CID_615 | DSSTox_GSID_20615 | DSSTox_RID_75691 |
| DTXSID9020615 | DivK1c_000147 | E 0516 |
| E0858 | EU-0100479 | Edolan |
| Etodlic Acid | Etodolac (JP17/USP/INN) | Etodolac [USAN:INN:BAN] |
| Etodolac [USAN:USP:INN:BAN] | Etodolac for peak identification, European Pharmacopoeia (EP) Reference Standard | Etodolac(Lodine) |
| Etodolac, European Pharmacopoeia (EP) Reference Standard | Etodolac, United States Pharmacopeia (USP) Reference Standard | Etodolac,(S) |
| Etodolaco | Etodolaco [INN-Spanish] | Etodolacum |
| Etodolacum [INN-Latin] | Etodolic acid | Etodolsaure |
| F2173-0681 | FT-0607046 | GTPL7185 |
| HMS1568P03 | HMS1921B09 | HMS2092O20 |
| HMS2095P03 | HMS2231D04 | HMS3259P10 |
| HMS3261O20 | HMS3374G09 | HMS3712P03 |
| HMS500H09 | HY-76251 | Hypen |
| IDI1_000147 | K-8734 | KBio1_000147 |
| KBio2_001724 | KBio2_004292 | KBio2_006860 |
| KBio3_002358 | KBioGR_000680 | KBioSS_001724 |
| KS-0000000W | KS-1056 | LP00479 |
| LS-7393 | Lodine | Lodine (TN) |
| Lodine SR | Lodine XL | Lopac0_000479 |
| MFCD00133313 | MLS000028474 | MLS001077315 |
| MLS006011566 | NC00718 | NCGC00015399-03 |
| NCGC00015399-04 | NCGC00015399-05 | NCGC00015399-06 |
| NCGC00015399-07 | NCGC00015399-08 | NCGC00015399-09 |
| NCGC00015399-11 | NCGC00015399-12 | NCGC00015399-13 |
| NCGC00015399-15 | NCGC00015399-16 | NCGC00016849-01 |
| NCGC00089769-02 | NCGC00089769-03 | NCGC00089769-04 |
| NCGC00089769-05 | NCGC00089769-06 | NCGC00089769-07 |
| NCGC00259767-01 | NCGC00261164-01 | NINDS_000147 |
| NNYBQONXHNTVIJ-UHFFFAOYSA-N | NSC 282126 | NSC-282126 |
| NSC-757821 | NSC282126 | NSC757821 |
| Opera_ID_1774 | Osteluc | Pharmakon1600-01501005 |
| Prestwick0_000231 | Prestwick1_000231 | Prestwick2_000231 |
| Prestwick3_000231 | Prestwick_209 | Pyrano(3,4,b)indole-1-acetic acid, 1,3,4,9-tetrahydro-1,8-diethyl- |
| Pyrano(3,4-b)indole-1-acetic acid, 1,3,4,9-tetrahydro-1,8-diethyl- | Pyrano(3,4-b)indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro- | Pyrano(3,4-b)indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro-(+-)- |
| Pyrano[3, 1,8-diethyl-1,3,4,9-tetrahydro- | Pyrano[3,4-b]indole-1-acetic acid, 1,8-diethyl-1,3,4,9-tetrahydro- | Q-201099 |
| Q2465218 | RAK-591 | RTR-036749 |
| Ramodar | SAM002589968 | SBI-0050463.P003 |
| SCHEMBL3903 | SMR000058443 | SPBio_001374 |
| SPBio_002222 | SPECTRUM1501005 | SR-01000000100 |
| SR-01000000100-2 | SR-01000000100-4 | SR-01000000100-7 |
| Spectrum2_001387 | Spectrum3_001429 | Spectrum4_000410 |
| Spectrum5_001347 | Spectrum_001244 | TR-036749 |
| Tedolan | Tox21_110644 | Tox21_110644_1 |
| Tox21_202218 | Tox21_500479 | Ultradol |
| Z1820178466 | Zedolac | [1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl]acetic acid |
| etodolac | etodolac o |