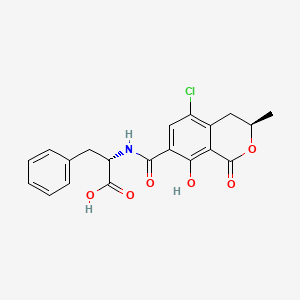
D0504 | ochratoxin a
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | rat | isolated liver mitochondria | increase | 66 | ||||
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | decrease | 66 | ||||
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 8 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H319 (37.5%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H330 (37.5%): Fatal if inhaled [Danger Acute toxicity, inhalation] H351 (100%): Suspected of causing cancer [Warning Carcinogenicity] H361 (50%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H362 (12.5%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H373 (12.5%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H413 (62.5%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P263, P264, P270, P271, P273, P280, P281, P284, P301+P310, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P330, P337+P313, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intravenous | 12750ug/kg (12.75mg/kg) | Annales Recherches Veterinaires. Vol. 5, Pg. 233, 1974. | |
| rat | LD50 | intraperitoneal | 12600ug/kg (12.6mg/kg) | Annales Recherches Veterinaires. Vol. 5, Pg. 233, 1974. | |
| rat | LD50 | oral | 20mg/kg (20mg/kg) | lungs, thorax, or respiration: chronic pulmonary edema | Food and Cosmetics Toxicology. Vol. 6, Pg. 479, 1968. |
| chicken | LDLo | subcutaneous | 11mg/kg (11mg/kg) | liver: "hepatitis (hepatocellular necrosis), zonal" | Applied Microbiology. Vol. 21, Pg. 492, 1971. |
| duck | LD50 | oral | 500ug/kg (0.5mg/kg) | liver: fatty liver degeration | Nature. Vol. 205, Pg. 1112, 1965. |
| mouse | LD50 | intraperitoneal | 22mg/kg (22mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 2, Pg. 109, 1946. | |
| turkey | LD50 | oral | 5900ug/kg (5.9mg/kg) | Poultry Science. Vol. 55, Pg. 786, 1976. | |
| domestic animals - goat/sheep | LD50 | intravenous | 1mg/kg (1mg/kg) | CRC Critical Reviews in Toxicology. Vol. 2, Pg. 499, 1974. | |
| rat | LD50 | unreported | 22mg/kg (22mg/kg) | Indian Journal of Experimental Biology. Vol. 29, Pg. 813, 1991. | |
| mouse | LD50 | oral | 46mg/kg (46mg/kg) | Toxicology Letters. Vol. 25, Pg. 1, 1985. | |
| mouse | LD50 | intravenous | 25710ug/kg (25.71mg/kg) | Annales Recherches Veterinaires. Vol. 5, Pg. 233, 1974. | |
| chicken | LD50 | oral | 3300ug/kg (3.3mg/kg) | Applied Microbiology. Vol. 21, Pg. 492, 1971. | |
| quail | LD50 | oral | 16500ug/kg (16.5mg/kg) | Poultry Science. Vol. 55, Pg. 786, 1976. | |
| (-)-N-((5-Chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenylalanine | (-)-N-((5-Chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl-3-phenylalanine | (2S)-2-[[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydroisochromene-7-carbonyl]amino]-3-phenylpropanoic acid |
| (2S)-2-{[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-carbonyl]amino}-3-phenylpropanoic acid | (2S)-2-{[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-yl]formamido}-3-phenylpropanoic acid | (R)-N-((5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1 H-2-benzopyran-7-yl)carbonyl)phenylalanine |
| (R)-N-((5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)-carbonyl)-L-phenylalanine | (R)-N-((5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl)phenylalanine | (R)-N-((5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-benzo(c)pyran-7-yl)carbonyl)-3-phenylalanine |
| (S)-2-((R)-5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-carboxamido)-3-phenylpropanoic acid | 1779SX6LUY | 2-[((3R)-5-chloro-8-hydroxy-3-methyl-1-oxoisochroman-7-yl)carbonylamino](2S)-3 -phenylpropanoic acid |
| 303-47-9 | 3R14S-Ochratoxin A | ACon0_000200 |
| ACon1_001268 | AKOS024456512 | Alanine, N-((5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-, (-)- |
| Alanine, N-[(5-chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl]-3-phenyl-, (-)- | Antibiotic 9663 | BIO1001 |
| BRD-K39944607-001-01-4 | BRN 1301486 | Bio1_000298 |
| Bio1_000787 | Bio1_001276 | C09955 |
| C20H18ClNO6 | CBiol_002012 | CCRIS 2382 |
| CHEBI:7719 | CHEMBL589366 | DTXSID7021073 |
| EINECS 206-143-7 | GTPL4672 | HSDB 4305 |
| InChI=1/C20H18ClNO6/c1-10-7-12-14(21)9-13(17(23)16(12)20(27)28-10)18(24)22-15(19(25)26)8-11-5-3-2-4-6-11/h2-6,9-10,15,23H,7-8H2,1H3,(H,22,24)(H,25,26 | J-017922 | L-Phenylalanine, N-((5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl)-, (R)- |
| L-Phenylalanine, N-[(5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl]-, (R)- | LS-1157 | MCULE-3363875457 |
| MEGxm0_000357 | MFCD00078079 | N-(((3R)-5-Chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-L-alanine |
| N-[(3R)-(5-Chloro-8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl]-L-phenylalanine | N-[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-carbonyl]-L-phenylalanine | N-[(5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl]phenylalanine, 9CI |
| N-[[(3R)-5-Chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl]carbonyl]-L-phenylalanine | N-{[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-2-benzopyran-7-yl]carbonyl}-L-phenylalanine | N-{[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl]carbonyl}-L-phenylalanine |
| NCGC00162403-01 | NCGC00162403-02 | NCGC00162403-03 |
| NCGC00162403-04 | NCGC00162403-05 | NCGC00162403-05_C20H18ClNO6_L-Phenylalanine, N-[[(3R)-5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl]carbonyl]- |
| NCI-C56586 | NSC 201422 | OCHRATOXIN A |
| OTA | Ochratoxin A 10 microg/mL in Acetonitrile | Ochratoxin A 10 microg/mL in Acetonitrile:Methanol |
| Ochratoxin A solution, 10 mug/mL in acetonitrile, analytical standard | Ochratoxin A solution, certified reference material, 50 mug/mL in benzene: acetic acid (99:1), ampule of 1 mL; | Ochratoxin A(OTA) |
| Ochratoxin A, 99+% | Ochratoxin A, aspergillus ochraceus | Ochratoxin A, from Petromyces albertensis, >=98% (HPLC) |
| Ochratoxin A, purum, >=98.0% (TLC), from Aspergillus ochraceus | Ochratoxin A, reference material, from Aspergillus ochraceus | Ochratoxin A-BSA conjugate from Aspergillus ochraceus |
| Phenylalanine - ochratoxin A | Q1885038 | RWQKHEORZBHNRI-BMIGLBTASA-N |
| SCHEMBL15105 | SMP2_000132 | ST50826330 |
| UNII-1779SX6LUY | ZINC3861782 | ZX-AFC000190 |
| ZX-AFC000204 |