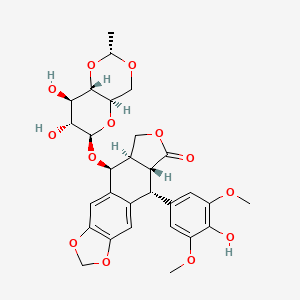
D0052 | Etoposide
L
L01CB01 Etoposide
[L01CB] Podophyllotoxin derivatives
[L01C] PLANT ALKALOIDS AND OTHER NATURAL PRODUCTS
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | > 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| MEMBRANE POTENTIAL | > 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| SWELLING | isolated mitochondria | increase | 57 | |||||
| ROS PRODUCTION | > 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| APOPTOSIS | isolated mitochondria | increase | 57 | |||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Reactive oxygen species | > 100 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| Cytochrome c | isolated mitochondria | release | 57 | |||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 200 companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 200 companies. For more detailed information, please visit ECHA C&L website Of the 7 notification(s) provided by 199 of 200 companies with hazard statement code(s): H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H350 (100%): May cause cancer [Danger Carcinogenicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H340: May cause genetic defects [Danger Germ cell mutagenicity] H350: May cause cancer [Danger Carcinogenicity] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H362: May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H371: May cause damage to organs [Warning Specific target organ toxicity, single exposure] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P263, P264, P270, P281, P301+P312, P308+P313, P309+P311, P314, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 15070ug/kg (15.07mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | subcutaneous | 143mg/kg (143mg/kg) | Drugs in Japan Vol. -, Pg. 190, 1990. | |
| women | TDLo | intravenous | 160ug/kg/3M-C (0.16mg/kg) | Lancet. Vol. 341, Pg. 1353, 1993. | |
| rabbit | LD50 | oral | 147mg/kg (147mg/kg) | Journal of Toxicological Sciences. Vol. 11(Suppl, | |
| mouse | LD50 | oral | 215mg/kg (215mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| rat | LD50 | intravenous | 58mg/kg (58mg/kg) | Drugs in Japan Vol. -, Pg. 230, 1995. | |
| rat | LD50 | oral | 1784mg/kg (1784mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 19, Pg. 3473, 1985. | |
| rat | LD50 | subcutaneous | > 200mg/kg (200mg/kg) | Drugs in Japan Vol. -, Pg. 190, 1990. | |
| man | TDLo | intravenous | 57ug/kg/2M-C (0.057mg/kg) | Lancet. Vol. 341, Pg. 1353, 1993. | |
| human | TDLo | intravenous | 2630ug/kg/10D (2.63mg/kg) | Cancer Vol. 34, Pg. 985, 1974. | |
| mouse | LD50 | intraperitoneal | 64mg/kg (64mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 19, Pg. 3473, 1985. | |
| rabbit | LD50 | intravenous | 37mg/kg (37mg/kg) | Journal of Toxicological Sciences. Vol. 11(Suppl, | |
| child | TDLo | intravenous | 183mg/kg/2H-C (183mg/kg) | behavioral: ataxia | Drug Intelligence and Clinical Pharmacy. Vol. 22, Pg. 41, 1988. |
| rat | LD50 | intraperitoneal | 39mg/kg (39mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 19, Pg. 3473, 1985. | |
| human | TDLo | oral | 16mg/kg/5D-I (16mg/kg) | Cancer Vol. 34, Pg. 985, 1974. | |
| (-)-Etoposide | (10R,11R,15R,16S)-16-{[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-hexahydro-2H-pyrano[3,2-d][1,3]dioxin-6-yl]oxy}-10-(4-hydroxy-3,5-dimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1(9),2,7-trien-12-one | (10r,11r,15r,16s)-16-([(2r,4ar,7r,8r,8as)-7,8-dihydroxy-2-methyl-hexahydro-2h-pyrano[3,2-d][1,3]diox |
| (5R,5aR,8aR,9S)-9-(((2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one | (5R,5aR,8aR,9S)-9-(((2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d] | (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxy-phenyl)-5a,6,8a,9-tetrahydro-5H-isobenzofuro[5,6-f][1,3]benzodioxol-8-one |
| (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f] | (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3'',4'':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside |
| (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside | 13165-EP2269989A1 | 13165-EP2270008A1 |
| 13165-EP2270014A1 | 13165-EP2270018A1 | 13165-EP2272827A1 |
| 13165-EP2272832A1 | 13165-EP2275413A1 | 13165-EP2275420A1 |
| 13165-EP2277565A2 | 13165-EP2277566A2 | 13165-EP2277567A1 |
| 13165-EP2277568A2 | 13165-EP2277569A2 | 13165-EP2277570A2 |
| 13165-EP2277865A1 | 13165-EP2277876A1 | 13165-EP2280012A2 |
| 13165-EP2281815A1 | 13165-EP2287156A1 | 13165-EP2289892A1 |
| 13165-EP2292280A1 | 13165-EP2292614A1 | 13165-EP2292615A1 |
| 13165-EP2292617A1 | 13165-EP2295055A2 | 13165-EP2295416A2 |
| 13165-EP2295426A1 | 13165-EP2295427A1 | 13165-EP2298305A1 |
| 13165-EP2298746A1 | 13165-EP2298748A2 | 13165-EP2298764A1 |
| 13165-EP2298765A1 | 13165-EP2298768A1 | 13165-EP2298772A1 |
| 13165-EP2298778A1 | 13165-EP2298780A1 | 13165-EP2301928A1 |
| 13165-EP2301933A1 | 13165-EP2305640A2 | 13165-EP2305642A2 |
| 13165-EP2305671A1 | 13165-EP2305679A1 | 13165-EP2305689A1 |
| 13165-EP2308812A2 | 13165-EP2308833A2 | 13165-EP2308839A1 |
| 13165-EP2308855A1 | 13165-EP2308861A1 | 13165-EP2311453A1 |
| 13165-EP2311807A1 | 13165-EP2311808A1 | 13165-EP2311825A1 |
| 13165-EP2311827A1 | 13165-EP2311829A1 | 13165-EP2311840A1 |
| 13165-EP2311842A2 | 13165-EP2314574A1 | 13165-EP2316832A1 |
| 13165-EP2316833A1 | 13165-EP2316834A1 | 13165-EP2374454A1 |
| 33419-42-0 | 4''-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside) | 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside |
| 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside) | 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside) | 4'-Demethylepipodophyllotoxin ethylidene-.beta.-D-glucoside |
| 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin | 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside | 419E420 |
| 6PLQ3CP4P3 | 9-((4,6-O-Ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, (5R-(5alpha,5abeta,8aalpha,9beta(R*)))- | 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4- hydroxy-3,4-dimethyloxyphenyl)furo (3',4'':6,7) naptho-(2,3-d)-1,3-dioxol-6 (5aH)-one |
| 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3'',4'''':6,7)naptho-(2,3-d)-1,3-dioxol-6(5aH)-one | 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3',4'':6,7)naptho-(2,3-d)-1,3-dioxol-6(5aH)-one | A-8109 |
| AB00438905 | AB00438905-17 | AB00438905-18 |
| AB00438905_19 | AB07572 | ACN-057122 |
| AKOS007930275 | AS-35312 | BCP9000669 |
| BDBM50127140 | BPBio1_000673 | BRD-K37798499-001-02-5 |
| BRD-K37798499-001-05-8 | BRD-K37798499-001-10-8 | BRD-K37798499-001-14-0 |
| BRD-K37798499-001-27-2 | BSPBio_000611 | C-23291 |
| C01576 | C29H32O13 | CC-28332 |
| CCG-101165 | CCRIS 2392 | CHEBI:4911 |
| CHEMBL44657 | CPD000112002 | CS-1774 |
| D00125 | DB00773 | Demethyl Epipodophyllotoxin Ethylidine Glucoside |
| Demethylepipodophyllotoxin-beta-D-ethylideneglucoside | EBD2157958 | EINECS 251-509-1 |
| EVP | EX-A1207 | Epipodophyllotoxin VP-16213 |
| Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside | Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI) | Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside) |
| Epipodophyllotoxin-beta-D-ethyliden-glucoside, 4'-demethyl- | Eposide | Etopol |
| Etopophos (phosphate salt) | Etoposide | Etoposide (JP17/USP/INN) |
| Etoposide (VP-16) | Etoposide (VP16) | Etoposide [USAN:INN:BAN:JAN] |
| Etoposide [USAN:USP:INN:BAN:JAN] | Etoposide for system suitability, European Pharmacopoeia (EP) Reference Standard | Etoposide, British Pharmacopoeia (BP) Reference Standard |
| Etoposide, European Pharmacopoeia (EP) Reference Standard | Etoposide, United States Pharmacopeia (USP) Reference Standard | Etoposide, synthetic, >=98%, powder |
| Etoposide,(S) | Etoposido | Etoposido [INN-Spanish] |
| Etoposidum | Etoposidum [INN-Latin] | Etosid |
| Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, 9-((4,6-O-(1R)-ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)- | Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, 9-((4,6-O-ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R-(5alpha,5abeta,8aalpha,9beta(R*)))- | Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one-, 9-((4,6-O-ethylidene-beta-D-glucopyranosyl)oxy)5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl), (5R-(5alpha,5abeta,8aalpha,9beta(R*)))- |
| Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-[[4,6-O-(1R)-ethylidene-.beta.-D-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)- | GTPL6815 | HMS2052N05 |
| HMS2089F14 | HMS2096O13 | HMS2232L03 |
| HMS3713O13 | HSDB 6517 | HY-13629 |
| LS-1214 | Lastet | MFCD00869325 |
| MLS000049957 | MLS001074951 | MLS001424283 |
| MLS002153463 | MLS002207239 | MLS002222184 |
| NC00415 | NCGC00179504-02 | NK 171 |
| NSC 141540 | NSC-141540 | NSC141540 |
| Prestwick3_000396 | Q418817 | SAM001246880 |
| SBI-0051910.P002 | SCHEMBL4259 | SMR000112002 |
| SR-01000763196 | SR-01000763196-3 | ST24047199 |
| Toposar | UNII-6PLQ3CP4P3 | VJJPUSNTGOMMGY-MRVIYFEKSA-N |
| VP 16 | VP 16 (pharmaceutical) | VP 16-213 |
| VP 16213 | VP-16 | VP-16 |
| VP-16-213 | VePESID (TN) | VePesid |
| Vepesid J | Vepeside | ZINC3938684 |
| Zuyeyidal | [1,3]benzodioxol-8-one | [1,3]dioxol-6(5aH)-one |
| [5R-[5?,5a?,8a?,9?(R*)]]-9-[(4,6-?-Ethylidene-?-D-glucopyranosyl)oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6-(5aH)-one | etoposide | s1225 |
| trans-Etoposide |