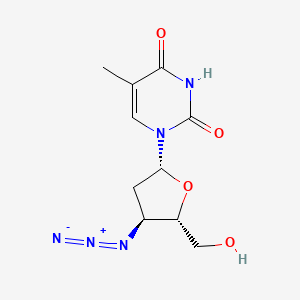
D0128 | Zidovudine
J
J05AR05 Zidovudine, lamivudine and nevirapine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR04 Zidovudine, lamivudine and abacavir
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR01 Zidovudine and lamivudine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AF01 Zidovudine
[J05AF] Nucleoside and nucleotide reverse transcriptase inhibitors
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 416.1 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | 242.0 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | increase | EC20 | 36 |
| ELECTRON TRANSPORT CHAIN | Interfere | 307 | ||||||
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 79μM | 83 | mice | Lean mice vs Ob/ob mice | Measurement of oxygen consumption in the presence of ADP (state 3) and the different substrates was carried out on the Mitologics screening platform | EC20 | 227 | |
| SWELLING | > 200 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| ULTRASTRUCTURE | abnormalize | 307 | ||||||
| ROS PRODUCTION | Increase | 307 | ||||||
| MITOCHONDRIAL DNA METABOLIC PROCESS | 194 | |||||||
| MITOCHONDRIAL DNA METABOLIC PROCESS | increase | 307 | ||||||
| MITOCHONDRIAL PROTEIN TRANSLATION | decrease | 307 | ||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Cytochrome c oxidase | Inhibition | 307 | ||||||
| Citrate synthase, mitochondrial | Inhibition | 307 | ||||||
| DNA polymerase gamma | 194 | |||||||
| Mitochondrial thymidine kinase 2 | human | U2OS cell | downregulate | 196 | ||||
| deoxyguanosine kinase | human | U2OS cell | downregulate | 196 | ||||
| Reactive oxygen species | increase | 307 | ||||||
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
Aggregated GHS information provided by 96 companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H341 (55.21%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H350 (54.17%): May cause cancer [Danger Carcinogenicity] H351 (44.79%): Suspected of causing cancer [Warning Carcinogenicity] H361 (55.21%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H372 (53.12%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P264, P270, P281, P308+P313, P314, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Danger |
H341: Suspected of causing genetic defects [Warning Germ cell mutagenicity] H351: Suspected of causing cancer [Warning Carcinogenicity] H361: Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H362: May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H370: Causes damage to organs [Danger Specific target organ toxicity, single exposure] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P263, P264, P270, P281, P307+P311, P308+P313, P314, P321, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione | (AZT)1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione | 1-((2R,4R,5S)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione |
| 1-((2R,4S,5S)-4-(diazoamino)-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione | 1-((2R,4S,5S)-4-AZIDO-5-(HYDROXYMETHYL)TETRAHYDROFURAN-2-YL)-5-METHYLPYRIMIDINE-2,4(1H,3H)-DIONE | 1-((2R,4S,5S)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione |
| 1-((2R,4S,5S)-4-azido-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione | 1-((2R,5S)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione | 1-((2S,4R,5R)-4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione |
| 1-(3-Azido-2,3-dideoxy-beta-D-ribofuranosyl)-5-methylpyrimidine-2,4-(1H,3H)-dione | 1-(3-Azido-2,3-dideoxy-beta-D-ribofuranosyl)thymine | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione |
| 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (AZT) | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (AzddThd, AZT) | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione (N3ddThd) |
| 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione [AZT] | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(3''-azido-2'',3''-dideoxythymidine) | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(AZT) |
| 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(Zidovudine, AZT) | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl-1H-pyrimidine-2,4-dione(azidothymidine, AZT) | 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione |
| 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione | 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione | 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-methylpyrimidine-2,4(1H,3H)-dione |
| 146426-54-2 | 3′-Azido-3′-deoxythymidine | 3''-Azido-3''-deoxy-thymidine |
| 3''-Deoxy-3-azidothymidine | 3''-azido-2'',3''-dideoxythymidine | 3''-azido-thymidine |
| 3''azido-2''3''-dideoxythymidine | 3'-Azido-2',3'-Dideoxythymidine | 3'-Azido-2',3'-dideoxythymidine & sCD4(soluble recombinant protein) |
| 3'-Azido-3'-deoxythymidine | 3'-Azido-3'-deoxythymidine & CD4-Pseudomonas exotoxin A hybrid | 3'-Azido-3'-deoxythymidine & Concanavalin A |
| 3'-Azido-3'-deoxythymidine & Erythropoietin | 3'-Azido-3'-deoxythymidine & Granulocyte-macrophage colony-stimulating factor | 3'-Azido-3'-deoxythymidine & Heteropolyoxotungstate PM-19 |
| 3'-Azido-3'-deoxythymidine & Interleukin-1 | 3'-Azido-3'-deoxythymidine & Interleukin-2 | 3'-Azido-3'-deoxythymidine & Interleukin-6 |
| 3'-Azido-3'-deoxythymidine & Lithium & Erythropoietin | 3'-Azido-3'-deoxythymidine & Lithium & Granulocyte-macrophage colony-stimulating factor | 3'-Azido-3'-deoxythymidine & Lithium & Interleukin-1 |
| 3'-Azido-3'-deoxythymidine & Lithium & Interleukin-6 | 3'-Azido-3'-deoxythymidine & Lymphoblastoid Interferon | 3'-Azido-3'-deoxythymidine & Recombinant Interferon-.alpha.-2 |
| 3'-Azido-3'-deoxythymidine & Sho-Saiko-To | 3'-Azido-3'-deoxythymidine (AIDS) | 3'-Azido-3'-deoxythymidine, >=98% (HPLC) |
| 3'-Azido-3'-deoxythymidine, >=99.0% (HPLC) | 3'-Azido-3'deoxythymidine & Interferon .alpha. | 3'-Azido-3'deoxythymidine & Nanoparticles (from human serum albumin or polyhexylcyanoacrylate) |
| 3'-Azido-3'deoxythymidine & Recombinant Soluble CD4 & Recombinant Interferon.alpha.A | 3'-Deoxy-3'-azidothymidine | 3'-azido-3'-deoxythymidine, AZT |
| 3'-azido3'-deoxythymidine | 3'-deoxy-3'-azido-thymidine | 3'azido-3'deoxythymidine |
| 3-((2S,3S,5R)-2-(hydroxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3-yl)triaz-1-en-2-ium-1-ide | 3-Azido-1,2,3-trideoxy-1-(3,4-dihydro-5-methyl-2,4-dioxo-1(2H)-pyrimidinyl)-D-erythro- pentofuranuronic acid | 3-Azido-3-deoxythymidine |
| 30516-87-1 | 399024-19-2 | 4-(4-Azido-5-hydroxy-tetrahydro-furan-2-yl)-5-methyl-3H-pyrazine-2,6-dione |
| 4B9XT59T7S | 4lhm | AB0012841 |
| AKOS005622576 | AKOS015842610 | ANW-26899 |
| AS-13019 | AZT | AZT & CD4(178)-PE 40 |
| AZT & Colony-stimulating factor 2 | AZT & Concanavalin A (ConA) | AZT & EPO |
| AZT & GM-CSF | AZT & HPA | AZT & IFN.alpha. |
| AZT & IFNL1 | AZT & IFNL2 | AZT & IFNL3 |
| AZT & IL-1 | AZT & IL-2 | AZT & IL-28A |
| AZT & IL-28B | AZT & IL-29 | AZT & IL-6 |
| AZT & Interferon lambda-1 | AZT & Interferon lambda-2 | AZT & Interferon lambda-3 |
| AZT & Interferon-.alpha.-2 | AZT & Interleukin 28A | AZT & Interleukin 28B |
| AZT & Interleukin 29 | AZT & Li & EPO | AZT & Li & GM-CSF |
| AZT & Li & IL-1 | AZT & Li & IL-6 | AZT & Lymphoblastoid Interferon |
| AZT & NP (from PHCA or HSA) | AZT & PM-19 | AZT & SST |
| AZT & rIFN.alpha.2 | AZT & rsCD4 & rIFN.alpha.A | AZT & rsT4 |
| AZT & sCD4 | AZT & srCD4 | AZT (Antiviral) |
| AZT Antiviral | AZT TRANSPLACENTAL CARCINOGENESIS STUDY | AZT+PRO 140 |
| AZT, Antiviral | Azidothymidine | Azidothymidine |
| Azidothymidine;AZT;ZDV | Aztec | BBL033764 |
| BDBM50002692 | BPBio1_000403 | BRD-K72903603-001-04-6 |
| BRD-K72903603-001-14-5 | BSPBio_000365 | BSPBio_003153 |
| BW A509U | BW-A 509U | BW-A-509U |
| BW-A509U | BWA-509U | BWA509U |
| Beta interferon(rIFN-beta seron) & 3'-Azido-3'-deoxythymidine(AZT) | C07210 | CCG-39924 |
| CCRIS 105 | CHEBI:10110 | CHEMBL129 |
| CPD000058351 | CTK4I2082 | CZ0012 |
| Certified Reference Material | Compound S | Cpd S |
| D00413 | DB00495 | DRG-0004 |
| DS-4152 & AZT | DSSTox_CID_127 | DSSTox_GSID_20127 |
| DSSTox_RID_75386 | DTXSID8020127 | DivK1c_000524 |
| EN300-52518 | FT-0601543 | GTPL4825 |
| HBOMLICNUCNMMY-XLPZGREQSA-N | HMS1921J20 | HMS2090G11 |
| HMS2092D06 | HMS2096C07 | HMS2234K17 |
| HMS3259H17 | HMS3713C07 | HMS501K06 |
| HSDB 6515 | IDI1_000524 | Interferon AD + 3'-azido-3'-deoxythymidine |
| Intron A & AZT | J-700147 | J10271 |
| K7 [P Ti2 W10 O40] | KBio1_000524 | KBio2_001828 |
| KBio2_004396 | KBio2_006964 | KBio3_002653 |
| KBioGR_000703 | KBioSS_001828 | KS-00000WOF |
| LS-1159 | Lecithinized superoxide dismutase & Thymidine, 3'-azido-3'-deoxy- | Liposomal AZT-SN-1 |
| Liposomal AZT-SN-3 | MCULE-8664408343 | MFCD00006536 |
| MLS000028548 | MLS001055351 | MLS001076358 |
| MLS002153202 | MLS002222249 | Met-SDF-1.beta. & AZT |
| Met-SDF-1.beta. & Zidovudine | Met-Stromal Cell-derived Factor-1.beta. (Human) & 3'-Azido-3'-deoxythymidine | NC00666 |
| NCGC00014918-01 | NCGC00023945-03 | NCGC00023945-04 |
| NCGC00023945-05 | NCGC00023945-06 | NCGC00023945-07 |
| NCGC00023945-08 | NCGC00023945-09 | NCGC00023945-10 |
| NCGC00023945-12 | NCGC00023945-13 | NCGC00178237-01 |
| NCGC00178237-02 | NCGC00254276-01 | NCGC00259752-01 |
| NINDS_000524 | NSC 602670 | NSC-758185 |
| NSC758185 | Opera_ID_1602 | PC-SOD+AZT |
| Pharmakon1600-01502109 | Prestwick3_000333 | Propolis & Thymidine, 3'-azido-3'-deoxy- |
| Propolis+AZT | Q198504 | Racemic Liposomal AZT |
| Retrovir | Retrovir (TN) | Retrovir(TM) |
| S2579 | SAM002548971 | SBI-0051731.P002 |
| SCHEMBL14615088 | SMR000058351 | SN-1-dipalmitoylglycerophospho-AZT (in a lipid vesicle) |
| SN-3-dipalmitoylglycerophospho-AZT (in a lipid vesicle) | SPBio_000834 | SPECTRUM1502109 |
| SR-01000000098 | SR-01000000098-3 | SR-05000001587 |
| SR-05000001587-1 | STK801891 | SW198799-2 |
| Spectrum2_000927 | Spectrum3_001507 | Spectrum4_000332 |
| Spectrum5_001101 | Spectrum_001348 | Sulfated polysaccharide-peptidoglycan DS-4152 & 3'-Azido-3'-deoxythymidine |
| Thymidine, 3'-azido-3'-deoxy- | Thymidine, 3'-azido-3'-deoxy- & PRO 140 (Anti-CCR5 monoclonal antibody) | Tox21_110062 |
| Tox21_110062_1 | Tox21_110894 | Tox21_202203 |
| Tox21_300578 | UNII-4B9XT59T7S | W-5037 |
| Z1723414428 | ZDV | ZIDOVUDINE [AZT] |
| ZINC3779042 | ZVD | Zidovudina |
| Zidovudina [Spanish] | Zidovudine | Zidovudine & IFNL1 |
| Zidovudine & IFNL2 | Zidovudine & IFNL3 | Zidovudine & IL-28A |
| Zidovudine & IL-28B | Zidovudine & IL-29 | Zidovudine & Interferon lambda-1 |
| Zidovudine & Interferon lambda-2 | Zidovudine & Interferon lambda-3 | Zidovudine & Interleukin 28A |
| Zidovudine & Interleukin 28B | Zidovudine & Interleukin 29 | Zidovudine (JP17/USP/INN) |
| Zidovudine (Retrovir) | Zidovudine [USAN:INN:BAN:JAN] | Zidovudine [USAN:USP:INN:BAN:JAN] |
| Zidovudine+PRO 140 | Zidovudine, European Pharmacopoeia (EP) Reference Standard | Zidovudine, Pharmaceutical Secondary Standard |
| Zidovudine, United States Pharmacopeia (USP) Reference Standard | Zidovudinum | Zidovudinum [Latin] |
| rIFN-beta seron & AZT | racemic-dipalmitoylglycerophospho-AZT (in a lipid vesicle) | zidovudin |
| zidovudine | zudovidine |
| DrugBank Name | Zidovudine |
| DrugBank | DB00495 |
| CAS Number | 146426-54-2, 25526-94-7, 30516-87-1, 399024-19-2 |
| PubChem Compound | 35370 |
| KEGG Compound ID | C07210 |
| KEGG Drug | D00413 |
| PubChem.Substance | 46508240 |
| ChEBI | 10110 |
| PharmGKB | PA451954 |
| ChemSpider | 32555 |
| BindingDB | 50002692.0 |
| TTD | DAP000701 |
| Wikipedia | Zidovudine |
| HET | AZZ |
| DPD | 1185 |