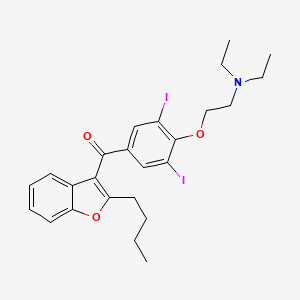
Drug
D0005 | Amiodarone
C
C01BD01 Amiodarone
[C01BD] Antiarrhythmics, class III
[C01B] ANTIARRHYTHMICS, CLASS I AND III
[C01] CARDIAC THERAPY
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | 10 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| UNCOUPLING | 1 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 10 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 20 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 50 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 80 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| UNCOUPLING | 100 μmol/L | rat; Sprague–Dawley | hepatocytes | Measurement of oxygen uptake | affect | p < 0.01 | 4 | |
| ELECTROPHORETIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | 0.5 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | Negative | p < 0.05 | 4 |
| MEMBRANE POTENTIAL | 1 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | Negative | p < 0.05 | 4 |
| MEMBRANE POTENTIAL | 4 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | Negative | p < 0.05 | 4 |
| MEMBRANE POTENTIAL | 8 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.05 | 4 |
| MEMBRANE POTENTIAL | 20 μmol/L | 1 hour | rat; Sprague–Dawley | hepatocytes | Measurement of mitochondrial membrane potential | decrease | p < 0.01 | 4 |
| MEMBRANE POTENTIAL | 2.6 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| MEMBRANE POTENTIAL | 50 µM | 1 hour | Human | HepG2 | High-content screening assay | Decrease | MEC | 306 |
| MEMBRANE POTENTIAL | 5 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| RESPIRATION | 45.92 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| STATE 3 RESPIRATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 20 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| STATE 3 RESPIRATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 20 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| STATE 3 RESPIRATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | Negative | p < 0.05 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 20 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 20 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| RESPIRATORY CONTROL RATIO (RCR) | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of oxygen uptake | decrease | p < 0.01 | 4 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | decrease | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | decrease | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | decrease | p < 0.001 | 3 | |
| ELECTRON TRANSPORT CHAIN | decrease | 36 | ||||||
| GLUCOSE GALACTOSE IC50 RATIO | 75.2 ± 12.9, 92.0 ± 5.5, 0.8, 95.1 ± 10.1 ,69.1 ± 24.1, 1.3 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with JC-1 mitochondrial membrane potential and ATP-depletion assays (CellTiter-Glo reagent ). | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla ) | 50 | ||
| FATTY ACID METABOLISM | 20µM | 24hr | isolated rat liver mitochondria, and the human hepatoma cell line HepG2 | inhibit | 271 | |||
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | Negative | p < 0.05 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 20 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 50 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 80 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | p < 0.01 | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of acyl‐CoA dehydrogenase activity | decrease | 28% inhibition | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of β‐ketothiolase activity | Negative | no inhibition | 4 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | affect | 227 | ||||||
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 228 μmol/L | rat | cardiac and hepatic mitochondria | CPT-1 activity was measured by the formation of palmitoyl-[3H]-carnitine from palmitoyl-CoA and [3H]-I- carnitine, | affect | IC50 | 240 | |
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 28.5/>100 | human/rat | hepatocytes | Fatty acid oxidation (FAO) was determined by measuring 14CO2 release from 14C-labeled palmitate | inhibition | IC50 (μM) | 333 | |
| SYNTHESIS OF KETONE BODY | 34 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of beta oxidation and ketone body formation | decrease | IC50 | 4 | |
| SYNTHESIS OF KETONE BODY | 16.6 | Wistar rat | hepatocytes | ketone bodies (KB = β-hydroxybutyrate + acetoacetate) were determined with a commercially available kit (Autokit 3-HB from Wako) | inhibition | IC50 (μM) | 333 | |
| SWELLING | 1 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | Negative | p < 0.05 | 4 | |
| SWELLING | 10 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | Negative | p < 0.05 | 4 | |
| SWELLING | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurements of mitochondrial swelling | increase | p < 0.05 | 4 | |
| SWELLING | ND | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | Negative | EC20 | 36 |
| OXIDATIVE STRESS | 0.1 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | Negative | p < 0.05 | 4 |
| OXIDATIVE STRESS | 1 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | p < 0.05 | 4 |
| OXIDATIVE STRESS | 100 μmol/L | 1 hour | human | HepG2 | Measurement of ROS | increase | p < 0.05 | 4 |
| OXIDATIVE STRESS | 185 | |||||||
| ROS PRODUCTION | 10 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| APOPTOSIS | 100 μmol/L | 8 hours | human | HepG2 | Assessment of cytochrome c release | increase | observable | 4 |
| LATE APOPTOSIS | 100 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | increase | p < 0.01 | 4 |
| LATE APOPTOSIS | 1 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | Negative | p < 0.05 | 4 |
| LATE APOPTOSIS | 100 μmol/L | 8 hours | rat; Sprague–Dawley | hepatocytes | Apoptosis measurement | increase | p < 0.05 | 4 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | inhibitor | p < 0.05 | 3 | |
| NADH:ubiquinone reductase | inhibitor | 36 | ||||||
| NADH:ubiquinone reductase | 45.92 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Succinate dehydrogenase | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| Quinol--cytochrome-c reductase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | inhibitor | p < 0.001 | 3 | |
| Cytochrome c oxidase | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | inhibitor | p < 0.05 | 3 | |
| ATP synthase | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | inhibitor | p < 0.001 | 3 | |
| carnitine palmitoyltransferases I | inhibit | 227 | ||||||
| carnitine palmitoyltransferases I | 228 μmol/L | rat | cardiac and hepatic mitochondria | CPT-1 activity was measured by the formation of palmitoyl-[3H]-carnitine from palmitoyl-CoA and [3H]-I- carnitine, | inhibit | IC50 | 240 | |
| Carnitine O-palmitoyltransferase 1, liver isoform, CPT1-L, EC 2.3.1.21 | >100/− | human/rat | recombinant Pichia pastoris Membrane Preparations | spectrophotometric assay with DTNB | Negative | IC50 (μM) | 333 | |
| Carnitine O-palmitoyltransferase 1, muscle isoform | >100 | human/rat | recombinant Pichia pastoris Membrane Preparations | spectrophotometric assay with DTNB | Negative | IC50 (μM) | 333 | |
| Carnitine O-palmitoyltransferase 2, mitochondrial, EC 2.3.1.21 | >100/− | human/rat | recombinant Pichia pastoris Membrane Preparations | spectrophotometric assay with DTNB | Negative | IC50 (μM) | 333 | |
| 3-ketoacyl-CoA thiolase, mitochondrial | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of β‐ketothiolase activity | Negative | no inhibition | 4 | |
| Short/branched chain specific acyl-CoA dehydrogenase, mitochondrial | 100 μmol/L | rat; Sprague–Dawley | liver mitochondria | Measurement of acyl‐CoA dehydrogenase activity | inhibitor | 28% inhibition | 4 | |
| Reactive oxygen species | 10 µM | 1 hour | Human | HepG2 | High-content screening assay | increase | MEC | 306 |
| Cytochrome c | 100 μmol/L | 8 hours | human | HepG2 | Assessment of cytochrome c release | release | observable | 4 |
| Cytochrome c | < 50 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | > 3gm/kg (3000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| mouse | LD50 | intraperitoneal | 450mg/kg (450mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| man | TDLo | unreported | 57mg/kg/5D-I (57mg/kg) | cardiac: pulse rate increase without fall in bp | Annals of Internal Medicine. Vol. 97, Pg. 561, 1982. |
| women | TDLo | multiple routes | 1475mg/kg/7W- (1475mg/kg) | cardiac: other changes | American Journal of Cardiology. Vol. 58, Pg. 1110, 1986. |
| women | TDLo | intravenous | 3mg/kg (3mg/kg) | brain and coverings: increased intracranial pressure | Critical Care Medicine. Vol. 13, Pg. 688, 1985. |
| man | TDLo | unreported | 51mg/kg/6D-C (51mg/kg) | skin and appendages (skin): "dermatitis, other: after systemic exposure" | British Medical Journal. Vol. 296, Pg. 1322, 1988. |
| child | LDLo | oral | 568mg/kg/9W-I (568mg/kg) | Journal of Pediatrics. Vol. 107, Pg. 967, 1985. | |
| women | TDLo | oral | 2480mg/kg/43W (2480mg/kg) | endocrine: thyroid weight (goiter) | Acta Medica Scandinavica. Vol. 221, Pg. 219, 1987. |
| rat | LD50 | intraperitoneal | 610mg/kg (610mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| man | LDLo | oral | 2086mg/kg/2Y- (2086mg/kg) | Netherlands Journal of Medicine. Vol. 29, Pg. 303, 1986. | |
| dog | LD50 | oral | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| man | TDLo | intravenous | 26mg/kg/1D-C (26mg/kg) | American Heart Journal. Vol. 131, Pg. 1214, 1996. | |
| women | TDLo | oral | 1128mg/kg/56W (1128mg/kg) | American Journal of Medicine. Vol. 86, Pg. 134, 1989. | |
| women | TDLo | oral | 64mg/kg/4D-I (64mg/kg) | Lancet. Vol. 350, Pg. 1300, 1997. | |
| women | TDLo | unreported | 48mg/kg/3D-I (48mg/kg) | cardiac: pulse rate increase without fall in bp | American Heart Journal. Vol. 130, Pg. 399, 1995. |
| dog | LD50 | intravenous | 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| women | TDLo | oral | 416mg/kg/30D- (416mg/kg) | lungs, thorax, or respiration: other changes | Archives of Internal Medicine. Vol. 147, Pg. 50, 1987. |
| man | TDLo | oral | 133mg/kg/23D- (133mg/kg) | skin and appendages (skin): photosensitivity: after systemic exposure | Lancet. Vol. 1, Pg. 51, 1984. |
| rat | LD50 | oral | > 3gm/kg (3000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| mouse | LD50 | oral | > 4gm/kg (4000mg/kg) | European Patent Application. Vol. #0076973, | |
| man | TDLo | oral | 171mg/kg/30D- (171mg/kg) | American Heart Journal. Vol. 100, Pg. 412, 1980. | |
| man | LDLo | unreported | 3650mg/kg/3.5 (3650mg/kg) | Clinical Endocrinology Vol. 45, Pg. 365, 1996. | |
| man | LDLo | oral | 3129mg/kg/3Y- (3129mg/kg) | Southern Medical Journal. Vol. 89, Pg. 85, 1996. | |
| man | TDLo | oral | 1714mg/kg/21W (1714mg/kg) | New England Journal of Medicine. Vol. 308, Pg. 779, 1983. | |
| rat | LD50 | intraperitoneal | 885mg/kg (885mg/kg) | European Patent Application. Vol. #0076973, | |
| women | TDLo | oral | 120mg/kg/10D- (120mg/kg) | cardiac: arrhythmias (including changes in conduction) | Human Toxicology. Vol. 4, Pg. 169, 1985. |
| man | LDLo | oral | 1869mg/kg/22W (1869mg/kg) | American Journal of Medicine. Vol. 77, Pg. 751, 1984. | |
| women | TDLo | oral | 5200mg/kg/5Y- (5200mg/kg) | Respiration. Vol. 49, Pg. 157, 1986. | |
| women | TDLo | oral | 5796mg/kg/69W (5796mg/kg) | Italian Journal of Neurological Sciences. Vol. 8, Pg. 605, 1987. | |
| man | TDLo | oral | 240mg/kg/6W-I (240mg/kg) | skin and appendages (skin): hair: other | Archives of Internal Medicine. Vol. 155, Pg. 1106, 1995. |
| rat | LD50 | intravenous | 170mg/kg (170mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| man | TDLo | oral | 3651mg/kg/2.3 (3651mg/kg) | American Journal of Gastroenterology. Vol. 83, Pg. 161, 1988. | |
| man | TDLo | oral | 60mg/kg/2W-I (60mg/kg) | Chest. Vol. 88, Pg. 630, 1985. | |
| man | TDLo | oral | 390mg/kg/39W- (390mg/kg) | Netherlands Journal of Medicine. Vol. 42, Pg. 21, 1993. | |
| mouse | LD50 | intraperitoneal | 254mg/kg (254mg/kg) | European Journal of Toxicology and Environmental Hygiene. Vol. 8, Pg. 122, 1975. | |
| mouse | LD50 | intravenous | 178mg/kg (178mg/kg) | European Journal of Toxicology and Environmental Hygiene. Vol. 8, Pg. 188, 1975. | |
| women | TDLo | intravenous | 36mg/kg/1D-I (36mg/kg) | endocrine: evidence of thyroid hyperfunction | Israel Journal of Medical Sciences. Vol. 21, Pg. 165, 1985. |
| man | TDLo | intravenous | 4286ug/kg (4.286mg/kg) | brain and coverings: increased intracranial pressure | Critical Care Medicine. Vol. 13, Pg. 688, 1985. |
| (2-Butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diidophenyl)methanone | (2-Butyl-benzofuran-3-yl)(4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl)methanone # | (2-Butylbenzofuran-3-yl)(4-(2-(diethylamino)-ethoxy)-3,5-diiodophenyl)methanone |
| (2-Butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)methanone | (2-butyl-1-benzofuran-3-yl)(4-{[2-(diethylamino)ethyl]oxy}-3,5-diiodophenyl)methanone | (2-butyl-1-benzofuran-3-yl)-[4-(2-diethylaminoethoxy)-3,5-diiodophenyl]methanone |
| (2-butyl-1-benzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl}methanone | (2-butylbenzofuran-3-yl)-[4-(2-diethylaminoethoxy)-3,5-diiodo-phenyl]-methanone | (2-butylbenzofuran-3-yl)-[4-(2-diethylaminoethyloxy)-3,5-diiodo-phenyl]methanone |
| (2-n-butyl-3-benzofuranyl) [4-[2-(diethylamino)ethoxyl]-3,5-diiodophenyl] methanone | (2-n-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxyl]-3,5-diiodophenyl]methanone | (2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-diiodophenoxy}ethyl)diethylamine |
| 1951-25-3 | 2-Butyl-3-(3,5-diiodo-4-(.beta.-diethylaminoethoxy)benzoyl)benzofuran | 2-Butyl-3-(3,5-diiodo-4-(2-diethylaminoethoxy)benzoyl)benzofuran |
| 2-Butyl-3-(3,5-diiodo-4-(beta-diethylaminoethoxy)benzoyl)benzofuran | 2-Butyl-3-(4'-.beta.-N-diethylaminoethoxy-3',5'-diiodobenzoyl)benzofuran | 2-Butyl-3-(4'-beta-N-diethylaminoethoxy-3',5'-diiodobenzoyl)benzofuran |
| 2-Butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl ketone | 2-Butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone | 2-Butyl-3-benzofuranyl p-((2-diethylamino)ethoxy)-m,m-diiodophenyl ketone |
| 2-Butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone | 2-n-Butyl-3',5'-diiodo-4'-N-diethylaminoethoxy-3-benzoylbenzofuran | 20514-EP2272832A1 |
| 20514-EP2305668A1 | 20514-EP2314585A1 | 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl 2-butylbenzo[b]furan-3-yl ketone |
| 5-18-02-00353 (Beilstein Handbook Reference) | 951A253 | AB00053422 |
| AB00053422-17 | AB00053422_18 | AB00053422_19 |
| AKOS005462717 | API0001453 | AX8114028 |
| Amidorone | Amiodarona | Amiodarona [INN-Spanish] |
| Amiodarone (USAN/INN) | Amiodarone Base | Amiodarone [USAN:BAN:INN] |
| Amiodarone [USAN:INN:BAN] | AmiodaroneHCl | Amiodaronum |
| Amiodaronum [INN-Latin] | Amjodaronum | Atlansil |
| BBI | BDBM18957 | BIDD:GT0425 |
| BIDD:PXR0146 | BPBio1_000372 | BRD-K17561142-003-16-8 |
| BRN 1271711 | BSPBio_000338 | BSPBio_001574 |
| BSPBio_002580 | Bio1_000026 | Bio1_000515 |
| Bio1_001004 | Bio2_000294 | Bio2_000774 |
| C-23400 | C06823 | C25H29I2NO3 |
| CAS-1951-25-3 | CAS-19774-82-4 | CBiol_001740 |
| CC-24083 | CCG-204217 | CCRIS 9360 |
| CHEBI:2663 | CHEMBL633 | CS-2834 |
| CTK4G7797 | Cordarone | Cordarone (Salt/Mix) |
| Cyto8E2 | D02910 | DB01118 |
| DSSTox_CID_2592 | DSSTox_GSID_22592 | DSSTox_RID_76649 |
| DTXSID7022592 | DivK1c_000079 | EINECS 217-772-1 |
| F2173-1018 | FT-0601533 | GTPL2566 |
| HMS1791O16 | HMS1989O16 | HMS2089C07 |
| HY-14187 | IDI1_000079 | IDI1_034044 |
| IYIKLHRQXLHMJQ-UHFFFAOYSA-N | KBio1_000079 | KBio2_000294 |
| KBio2_000741 | KBio2_002862 | KBio2_003309 |
| KBio2_005430 | KBio2_005877 | KBio3_000587 |
| KBio3_000588 | KBio3_001800 | KBioGR_000294 |
| KBioGR_001859 | KBioSS_000294 | KBioSS_000741 |
| Ketone, 2-butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl | Kordaron | L 3428 |
| L-3428 | L001174 | LS-87088 |
| Labaz (Salt/Mix) | Lopac-A-8423 | Lopac0_000122 |
| MCULE-4156227717 | Methanone, (2-butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)- | Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]- |
| Methanone,(2-butyl-3-benzofuranyl)[4-[2-(diethyloxidoamino)ethoxy]-3,5-diiodophenyl]- | N3RQ532IUT | NCGC00015096-01 |
| NCGC00015096-02 | NCGC00015096-03 | NCGC00015096-04 |
| NCGC00015096-05 | NCGC00015096-06 | NCGC00015096-07 |
| NCGC00015096-08 | NCGC00015096-09 | NCGC00015096-10 |
| NCGC00015096-11 | NCGC00015096-12 | NCGC00015096-13 |
| NCGC00015096-14 | NCGC00015096-17 | NCGC00024242-03 |
| NCGC00024242-04 | NCGC00024242-05 | NCGC00024242-06 |
| NCI60_041885 | NINDS_000079 | Pacerone |
| Prestwick0_000409 | Prestwick1_000409 | Prestwick2_000409 |
| Prestwick3_000409 | Q410061 | QTL1_000008 |
| SBI-0050110.P003 | SC-18813 | SCHEMBL16284 |
| SKF 33134-A | SKF-33134-A (Salt/Mix) | SKF-33134A |
| SPBio_001825 | SPBio_002277 | ST51014902 |
| STK529812 | Spectrum2_001813 | Spectrum3_001050 |
| Spectrum4_001190 | Spectrum5_001533 | Spectrum_000261 |
| Tox21_110083 | Tox21_110083_1 | Tranquerone |
| UNII-N3RQ532IUT | UNM000001215003 | W-107695 |
| ZINC3830212 | amiodarone | cid_441325 |
| DrugBank Name | Amiodarone |
| DrugBank | DB01118 |
| CAS Number | 1951-25-3, 19774-82-4, 318267-30-0 |
| PubChem Compound | 2157 |
| KEGG Compound ID | C06823 |
| KEGG Drug | D02910 |
| PubChem.Substance | 46507387 |
| ChEBI | 2663 |
| PharmGKB | PA448383 |
| ChemSpider | 2072 |
| BindingDB | 18957.0 |
| TTD | DAP000496 |
| Wikipedia | Amiodarone |
| HET | BBI |
| DPD | 1400 |
1. Chan et al. (2005)
2. Dykens et al. (2007)