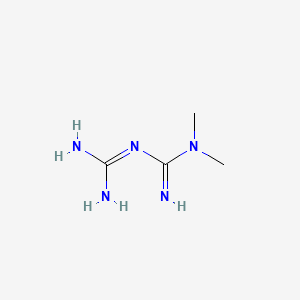
D0080 | Metformin
A
A10BD25 Metformin, saxagliptin and dapagliflozin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD23 Metformin and ertugliflozin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD22 Metformin and evogliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD20 Metformin and empagliflozin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD18 Metformin and gemigliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD17 Metformin and acarbose
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD16 Metformin and canagliflozin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD15 Metformin and dapagliflozin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD14 Metformin and repaglinide
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD13 Metformin and alogliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD11 Metformin and linagliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD10 Metformin and saxagliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD08 Metformin and vildagliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD07 Metformin and sitagliptin
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD05 Metformin and pioglitazone
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD03 Metformin and rosiglitazone
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BD02 Metformin and sulfonylureas
[A10BD] Combinations of oral blood glucose lowering drugs
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
A10BA02 Metformin
[A10BA] Biguanides
[A10B] BLOOD GLUCOSE LOWERING DRUGS, EXCL. INSULINS
[A10] DRUGS USED IN DIABETES
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 500 μM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | Negative | 15 | |
| MEMBRANE POTENTIAL | 1 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | decrease | 15 | |
| MEMBRANE POTENTIAL | 2 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | decrease | 15 | |
| MEMBRANE POTENTIAL | 388.4 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | > 400 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | 351.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| STATE 2 RESPIRATION | 500 nmol/mg mitochondrial protein | rat; Sprague–Dawley | liver mitochondria | Meassurement of respiration | Negative | 15 | ||
| STATE 2 RESPIRATION | 500 nmol/mg mitochondrial protein | 40 minutes preincubation | rat; Sprague–Dawley | liver mitochondria | Meassurement of respiration | decrease | 15 | |
| STATE 3 RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex I substrate; added ADP (500 μM) | decrease | p < 0.05 | 172 |
| STATE 3 RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex II substrate; added ADP (500 μM) | Negative | p < 0.05 | 172 |
| STATE 3 RESPIRATION | 500 nmol/mg mitochondrial protein | rat; Sprague–Dawley | liver mitochondria | Meassurement of respiration | Negative | 15 | ||
| STATE 3 RESPIRATION | 500 nmol/mg mitochondrial protein | 40 min preincubation | rat; Sprague–Dawley | liver mitochondria | Meassurement of respiration | decrease | 15 | |
| STATE 4 RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex I substrate; added oligomycin (2.5 μg oligomycin/mg mitochondrial protein) | decrease | p < 0.05 | 172 |
| STATE 4 RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex II substrate; added oligomycin (2.5 μg oligomycin/mg mitochondrial protein) | Negative | p < 0.05 | 172 |
| OXYGEN CONSUMPTION RATE (OCR) | 500 μM | 24 hours | human | HepG2 cells | Measurement of OCR | Negative | p < 0.001 | 15 |
| MAXIMAL RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex I substrate; added FCCP (1.5 μM) | decrease | p < 0.05 | 172 |
| MAXIMAL RESPIRATION | 2mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Measurement of Oxygen level; incubated in complex II substrate; added FCCP (1.5 μM) | Negative | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | Negative | p < 0.05 | 3 | |
| ELECTRON TRANSPORT CHAIN | 0.05 mM | 24 hours | human | MCF7 | Meassurement of mitochondrial respiration | Negative | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | human | MCF7 | Meassurement of mitochondrial respiration | Negative | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 5 mM | 24 hours | human | MCF7 | Meassurement of mitochondrial respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.05 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration | Negative | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 5 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | mouse | NMuMG | Meassurement of mitochondrial respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | mouse | NT2196 | Meassurement of mitochondrial respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | human | MCF10A | Meassurement of mitochondrial respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 0.5 mM | 24 hours | human | MCF7 | Meassurement of mitochondrial respiration | decrease | p < 0.05 | 172 |
| ELECTRON TRANSPORT CHAIN | 66 mM | Bovine | heart mitochondria | Measurement of complex I activity | decrease | IC50 | 15 | |
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | inhibit | 197 | ||||||
| UNCOUPLED RESPIRATION | 0.05 mM | 24 hours | human | MCF7 | Meassurement of uncoupled respiration | Negative | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF7 | Meassurement of uncoupled respiration | Negative | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 5 mM | 24 hours | human | MCF7 | Meassurement of uncoupled respiration | Negative | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.05 mM | 24 hours | human | MCF7 | Meassurement of non-mitochondrial respiration | Negative | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF7 | Meassurement of non-mitochondrial respiration | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 5 mM | 24 hours | human | MCF7 | Meassurement of non-mitochondrial respiration | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration; Meassurement of uncoupled respiration; cells grown in glucose | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration; Meassurement of uncoupled respiration; cells grown in galactose | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | mouse | NMuMG | Meassurement of coupled respiration; Meassurement of uncoupled respiration | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | mouse | NT2196 | Meassurement of coupled respiration; Meassurement of uncoupled respiration | Negative | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF10A | Meassurement of coupled respiration; Meassurement of uncoupled respiration | increase | p < 0.05 | 172 |
| UNCOUPLED RESPIRATION | 0.5 mM | 24 hours | human | MCF7 | Meassurement of coupled respiration; Meassurement of uncoupled respiration | Negative | p < 0.05 | 172 |
| GENERATION OF LACTATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of lactate concentration | Negative | p < 0.05 | 172 |
| GENERATION OF LACTATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE TO PYRUVATE RATIO | 0.5mM | 24 hours | human | MCF7 | Meassurement of lactate + pyruvate concentration; compared with MCF10A | increase | p < 0.05 | 172 |
| LACTATE TO PYRUVATE RATIO | 5.0mM | 24 hours | human | MCF7 | Meassurement of lactate + pyruvate concentration; compared with MCF10A | increase | p < 0.05 | 172 |
| GENERATION OF CITRATE | 0.5mM | 24 hours | human | MCF10A | Meassurement of citrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF CITRATE | 0.5mM | 24 hours | human | MCF7 | Meassurement of citrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF CITRATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of citrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF CITRATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of citrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ISOCITRATE | 0.5mM | 24 hours | human | MCF10A | Meassurement of isocitrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ISOCITRATE | 0.5mM | 24 hours | human | MCF7 | Meassurement of isocitrate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ALPHA-KETOGLUTARATE | 0.5mM | 24 hours | human | MCF10A | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ALPHA-KETOGLUTARATE | 0.5mM | 24 hours | human | MCF7 | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ALPHA-KETOGLUTARATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF ALPHA-KETOGLUTARATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | affect | p < 0.05 | 172 |
| GENERATION OF SUCCINATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of succinate concentration | decrease | p < 0.05 | 172 |
| GENERATION OF SUCCINATE | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of succinate concentration | decrease | p < 0.05 | 172 |
| ECAR | 500 μM | 24 hours | human | HepG2 cells | Measurement of ECAR | Negative | p < 0.001 | 15 |
| GLUCOSE CONSUMPTION | 5 mM | 24 hours | mouse | NMuMG | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 24 hours | mouse | NT2196 | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 24 hours | human | MCF10A | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 24 hours | human | MCF7 | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 48 hours | mouse | NMuMG | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 48 hours | mouse | NT2196 | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 48 hours | human | MCF10A | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| GLUCOSE CONSUMPTION | 5 mM | 48 hours | human | MCF7 | Meassurement of glucose concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 24 hours | mouse | NMuMG | Meassurement of lactate concentration | Negative | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 24 hours | mouse | NT2196 | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 24 hours | human | MCF10A | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 24 hours | human | MCF7 | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 48 hours | mouse | NMuMG | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 48 hours | mouse | NT2196 | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 48 hours | human | MCF10A | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| LACTATE PRODUCTION | 5 mM | 48 hours | human | MCF7 | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| ATP SYNTHESIS | 62.5 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | Negative | p < 0.001 | 15 |
| ATP SYNTHESIS | 62.5 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | Negative | p < 0.001 | 15 |
| ATP SYNTHESIS | 657 μM | 24 hours | human | hepatocytes | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| ATP SYNTHESIS | 1430 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| ATP SYNTHESIS | > 2000 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| SWELLING | > 400 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| OXIDATIVE STRESS | 500 μM | 24 hours | human | hepatocytes | ROS measurement | Negative | 15 | |
| OXIDATIVE STRESS | 1 mM | 24 hours | human | hepatocytes | ROS measurement | increase | 15 | |
| OXIDATIVE STRESS | 2 mM | 24 hours | human | hepatocytes | ROS measurement | increase | 15 | |
| OXIDATIVE STRESS | 500 μM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | Negative | 15 | |
| OXIDATIVE STRESS | 1 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | affect | 15 | |
| OXIDATIVE STRESS | 2 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | affect | 15 | |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 50 μM | bovine | heart mitochondria | Measurement of complex I activity | Negative | p < 0.05 | 3 | |
| NADH:ubiquinone reductase | 66 mM | Bovine | heart mitochondria | Measurement of complex I activity | inhibitor | IC50 | 15 | |
| NADH:ubiquinone reductase | inhibitor | 35 | ||||||
| NADH:ubiquinone reductase | > 400 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| Succinate dehydrogenase | 351.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Quinol--cytochrome-c reductase | 50 μM | bovine | heart mitochondria | Measurement of complex II + III activity | Negative | p < 0.05 | 3 | |
| Cytochrome c oxidase | 50 μM | bovine | heart mitochondria | Measurement of complex IV activity | Negative | p < 0.05 | 3 | |
| ATP synthase | 50 μM | bovine | heart mitochondria | Measurement of complex V activity | Negative | p < 0.05 | 3 | |
| ATP | 62.5 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | Negative | p < 0.001 | 15 |
| ATP | 62.5 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | Negative | p < 0.001 | 15 |
| ATP | 657 μM | 24 hours | human | hepatocytes | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| ATP | 1430 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| ATP | > 2000 μM | 24 hours | human | HepG2 cells | Assay of Cellular ATP Content | decrease | IC50 | 15 |
| lactate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of lactate concentration | Negative | p < 0.05 | 172 |
| lactate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of lactate concentration | increase | p < 0.05 | 172 |
| citrate | 0.5mM | 24 hours | human | MCF10A | Meassurement of citrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| citrate | 0.5mM | 24 hours | human | MCF7 | Meassurement of citrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| citrate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of citrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| citrate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of citrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| isocitrate | 0.5mM | 24 hours | human | MCF10A | Meassurement of isocitrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| isocitrate | 0.5mM | 24 hours | human | MCF7 | Meassurement of isocitrate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| alpha-ketoglutarate | 0.5mM | 24 hours | human | MCF10A | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| alpha-ketoglutarate | 0.5mM | 24 hours | human | MCF7 | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| alpha-ketoglutarate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| alpha-ketoglutarate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of alpha-ketoglutarate concentration; compared with MCF10A | decrease | p < 0.05 | 172 |
| succinate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of succinate concentration | decrease | p < 0.05 | 172 |
| succinate | 5mM | 30 minutes | mouse; C57BL/6J | isolated skeletal muscle mitochondria | Meassurement of succinate concentration | decrease | p < 0.05 | 172 |
| Reactive oxygen species | 500 μM | 24 hours | human | hepatocytes | ROS measurement | Negative | 15 | |
| Reactive oxygen species | 1 mM | 24 hours | human | hepatocytes | ROS measurement | increase | 15 | |
| Reactive oxygen species | 2 mM | 24 hours | human | hepatocytes | ROS measurement | increase | 15 | |
| oxidized glutathione | 500 μM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | Negative | 15 | |
| oxidized glutathione | 1 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | decrease | 15 | |
| oxidized glutathione | 2 mM | 24 hours | human | hepatocytes | Measurement of mitochondrial membrane potential | decrease | 15 | |
| Cytochrome c | > 800 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 3 companies from 2 notifications to the ECHA C&L Inventory. Reported as not meeting GHS hazard criteria by 2 of 3 companies. For more detailed information, please visit ECHA C&L website Of the 1 notification(s) provided by 1 of 3 companies with hazard statement code(s): H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Warning |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
P264, P270, P280, P301+P312, P302+P352, P305+P351+P338, P321, P330, P332+P313, P337+P313, P362, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 4gm/kg (4000mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 8, Pg. 329, 1952. | |
| mouse | LD50 | oral | > 10gm/kg (10000mg/kg) | Toksikologicheskii Vestnik. Vol. (4), Pg. 39, 1998. | |
| rat | LDLo | unreported | 600mg/kg (600mg/kg) | Biochemical Pharmacology. Vol. 14, Pg. 1325, 1965. | |
| rat | LD | oral | > 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 17, 1953. | |
| rabbit | LD50 | intraperitoneal | > 3gm/kg (3000mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 8, Pg. 329, 1952. | |
| 1,1-Dimethyl biguanide | 1,1-Dimethylbiguanide | 1-[(E)-amino(dimethylamino)methylidene]guanidine |
| 1-[(diaminomethylidene)amino]-N,N-dimethylmethanimidamide | 1-carbamimidamido-N,N-dimethylmethanimidamide | 15673-EP2269977A2 |
| 15673-EP2269989A1 | 15673-EP2270007A1 | 15673-EP2270011A1 |
| 15673-EP2272825A2 | 15673-EP2272834A1 | 15673-EP2272841A1 |
| 15673-EP2275108A1 | 15673-EP2275414A1 | 15673-EP2280001A1 |
| 15673-EP2287165A2 | 15673-EP2287166A2 | 15673-EP2292228A1 |
| 15673-EP2292620A2 | 15673-EP2295406A1 | 15673-EP2295409A1 |
| 15673-EP2295411A1 | 15673-EP2295422A2 | 15673-EP2298742A1 |
| 15673-EP2298769A1 | 15673-EP2298772A1 | 15673-EP2298776A1 |
| 15673-EP2298779A1 | 15673-EP2301533A1 | 15673-EP2301923A1 |
| 15673-EP2301929A1 | 15673-EP2301935A1 | 15673-EP2301936A1 |
| 15673-EP2305648A1 | 15673-EP2305674A1 | 15673-EP2308839A1 |
| 15673-EP2308847A1 | 15673-EP2308878A2 | 15673-EP2314576A1 |
| 15673-EP2314588A1 | 15673-EP2316470A2 | 3-(diaminomethylene)-1,1-dimethyl-guanidine;hydrochloride |
| 3-(diaminomethylidene)-1,1-dimethylguanidine | 3-(diaminomethylidene)-1,1-dimethylguanidine;hydrochloride | 3-[bis(azanyl)methylidene]-1,1-dimethyl-guanidine;hydrochloride |
| 3-carbamimidoyl-1,1-dimethyl-guanidine | 657-24-9 | 657M249 |
| 9100L32L2N | A19551 | AB2000590 |
| AKOS000121065 | AKOS005206848 | AKOS015966566 |
| AS-65365 | BBL012337 | BDBM50229665 |
| BDBM57047 | BIDD:GT0697 | BIGUANIDE, 1,1-DIMETHYL- |
| BPBio1_000009 | BRD-K79602928-003-04-1 | BRD-K79602928-003-08-2 |
| BSPBio_000007 | BSPBio_002314 | C07151 |
| C4H11N5 | CAS-1115-70-4 | CAS-657-24-9 |
| CCG-102605 | CCRIS 9321 | CHEBI:6801 |
| CHEMBL1431 | CS-0009563 | CTK2F2882 |
| D04966 | DB00331 | DMBG |
| DMGG | DSSTox_CID_3270 | DSSTox_GSID_23270 |
| DSSTox_RID_76950 | DTXSID2023270 | Diabex |
| Dianben | Dimethylbiguanid | Dimethylbiguanide |
| Dimethyldiguanide | Dimethylguanylguanidine | EINECS 211-517-8 |
| FT-0628266 | Fluamine | Flumamine |
| GTPL4503 | GTPL4779 | Glifage |
| Gliguanid | Glucophage | Glucophage (Salt/Mix) |
| Glumetza | HMS2089D19 | HSCI1_000295 |
| Haurymelin | Imidodicarbonimidic diamide, N,N-dimethyl- | Imidodicarbonimidic diamide-, N,N-dimethyl- |
| Imidodicarbonimidicdiamide, N,N-dimethyl- | Islotin | K070 |
| KBio2_002310 | KBio2_004878 | KBio2_007446 |
| KBio3_002790 | KBioGR_002310 | KBioSS_002312 |
| LA 6023 (Salt/Mix) | LA-6023 | LS-43899 |
| MCULE-7393156510 | MF8 | MLS000028493 |
| Melbin | Metformin (USAN/INN) | Metformin [USAN:INN:BAN] |
| Metformin base | Metformina | Metformina [DCIT] |
| Metformina [Spanish] | Metformine | Metformine [INN-French] |
| Metforminum | Metforminum [INN-Latin] | Metiguanide |
| N,N-Dimethylbiguanide | N,N-Dimethyldiguanide | N,N-Dimethylguanylguanidin |
| N,N-dimethylimidodicarbonimidic diamide | N-dimethylbiguanide | N1,N1-Dimethylbiguanide |
| NCGC00016564-01 | NCGC00016564-02 | NCGC00016564-03 |
| NCGC00016564-05 | NCGC00016564-07 | NCGC00188959-01 |
| NCGC00255255-01 | NNDG | Obimet |
| Prestwick0_000004 | Prestwick1_000004 | Prestwick2_000004 |
| Prestwick3_000004 | Q19484 | SBI-0206876.P001 |
| SC-73311 | SCHEMBL10276396 | SCHEMBL8944 |
| SCHEMBL9913821 | SMR000058277 | SPBio_001928 |
| STK011633 | STL483693 | STL484070 |
| Siofor | Tox21_302370 | UNII-9100L32L2N |
| W-109589 | XZWYZXLIPXDOLR-UHFFFAOYSA-N | ZINC12859773 |
| [14C]-metformin | [14C]metformin | cMAP_000016 |
| cid_14219 | metformin | n',n'-dimethylbiguanide |
| {[amino(dimethylamino)methylidene]amino}methanimidamide |
| DrugBank Name | Metformin |
| DrugBank | DB00331 |
| CAS Number | 1115-70-4, 461-58-5, 4931-70-8, 657-24-9 |
| PubChem Compound | 4091 |
| KEGG Compound ID | C07151 |
| KEGG Drug | D04966 |
| PubChem.Substance | 46507752 |
| ChEBI | 6801 |
| PharmGKB | PA450395 |
| ChemSpider | 3949 |
| BindingDB | 50229665.0 |
| TTD | DAP000205 |
| Wikipedia | Metformin |
| HET | MF8 |
| DPD | 10147 |